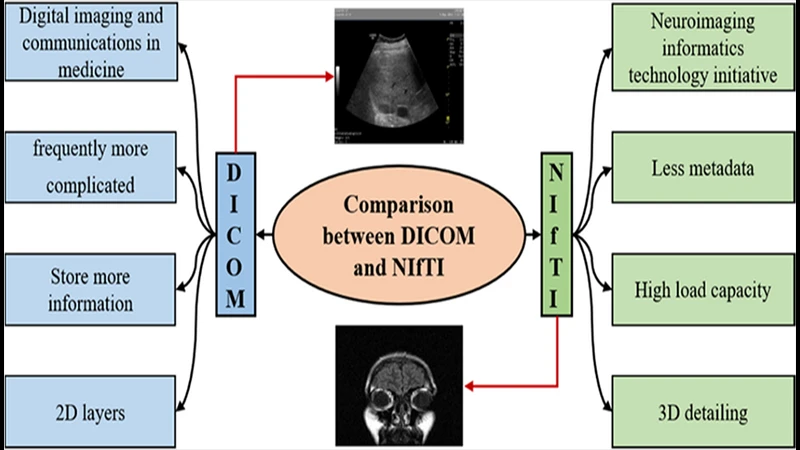
DICOM vs. NIfTI: Understanding the Key Differences for Neuroimaging
While DICOM is the undisputed standard for clinical medical imaging, anyone entering the world of academic neuroimaging research will quickly encounter another format: NIfTI. Though both are used to store brain scans, they were designed for very different purposes. Understanding their core differences is key to working effectively in both clinical and research environments.
DICOM: The Clinical Standard
DICOM (Digital Imaging and Communications in Medicine) is designed for the complex workflow of a hospital. Its primary focus is on patient data integrity and interoperability between different manufacturers' equipment.
- Structure: A single MRI study can consist of hundreds or even thousands of individual DICOM files, with each file representing a single 2D slice.
- Metadata: Its greatest strength is its incredibly rich metadata, stored in hundreds of standardized DICOM tags. This includes extensive, legally required patient information (name, MRN, etc.), detailed study parameters, and scanner information.
- Primary Goal: To ensure a medical image is inextricably linked to the correct patient and clinical context. Patient data safety is paramount.
NIfTI: The Research Standard
NIfTI (Neuroimaging Informatics Technology Initiative) was created by researchers, for researchers. Its primary focus is on simplifying data analysis, particularly for 3D and 4D brain imaging data (like fMRI).
- Structure: A single NIfTI file (.nii or .nii.gz) contains the entire 3D (or 4D) volume of a scan. All the slices of a brain MRI, for example, are stored together in one file.
- Metadata: The NIfTI header is much simpler than DICOM's. It contains essential information for analysis, such as image dimensions, voxel size, and spatial orientation, but it strips out all protected patient health information (PHI).
- Primary Goal: To provide a simple, anonymized, and analysis-friendly format for sharing and processing neuroimaging data.
Why the Difference? The Research Workflow
The first step in any research study is to convert the raw clinical DICOM images into the NIfTI format. This is done for several key reasons:
- Anonymization: The conversion process strips out all patient-identifying tags, which is a requirement for sharing data and protecting patient privacy in research settings.
- Simplicity: It is vastly easier for analysis software (like FSL, SPM, or ANTs) to work with a single 3D/4D NIfTI file than to manage thousands of individual 2D DICOM slices.
- Spatial Orientation: The NIfTI header contains a robust system for defining the spatial orientation of the brain (which way is left, right, up, down, etc.). This is absolutely critical for accurately aligning and comparing brains from many different subjects in a group study.
At-a-Glance Comparison Table
| Feature | DICOM | NIfTI |
|---|---|---|
| Primary Use | Clinical Care & Diagnosis | Academic Research & Analysis |
| File Structure | Many files per scan (one per slice) | One file per scan (3D/4D volume) |
| Metadata | Very extensive, includes PHI | Simple, anonymized, analysis-focused |
| Anonymization | Requires special tools to do properly | Anonymized by design |
Conclusion: The Right Tool for the Job
Neither format is inherently "better"—they are simply optimized for different tasks. DICOM is the robust, patient-centric standard essential for the day-to-day operations and legal requirements of a hospital imaging department. NIfTI is the streamlined, analysis-ready format that has become the lingua franca of the brain imaging research community. Professionals who bridge these two worlds, such as clinical researchers, must be proficient in handling both and understanding the critical conversion process from one to the other.

Comments